Document management SaaS for pharmaceutical companies
Simplifies and organizes event management, document change, and versioning tracking in pharmaceutical manufacturing making all that user-friendly, easy and simple. Implemented as a SaaS system it eliminates any expenses for pharmaceutical companies connected with their own old-school software they still use today.
The technical stack includes:
The problem
Our client wanted to create one and only system that handles everything that happens with pharmaceutical documents within their endless life cycle. Pharmaceutical production companies use a combination of many CRM, ERP, document applications poorly integrated and not optimized for pharmaceutical specifics. Using them is difficult, they are very slow and non-intuitive. Alternatives are either unusable or cost a lot.
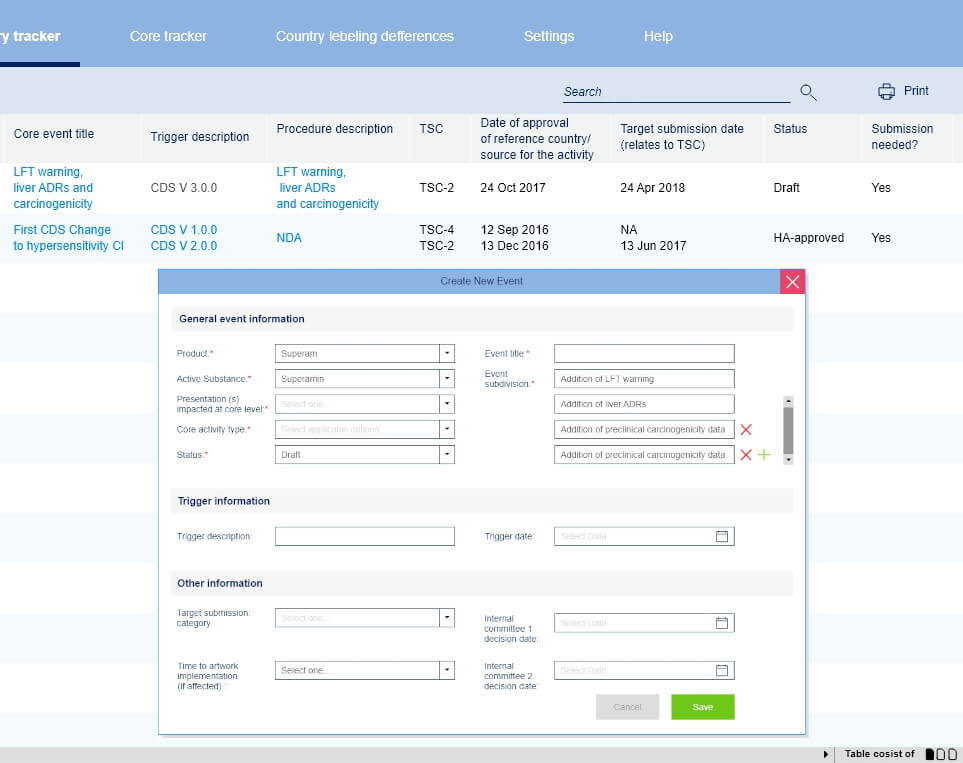
UI/UX challenge
The biggest part of employees in the pharmaceutical industry who manage document changes is 50+ years old. They still use and like standalone applications of the previous age and don't recognize modern web UX standards. They use an older version of MS Windows and don't update them. We created a web application that utilizes MS Windows UI style and all their favorite UX components such as trees, tables, etc. They accepted UI and workflow we created.
The solution
The goal was to develop a system that will horizontally pierce the whole manufacturing process and handles every stage, task, and state of any document minimizing human labor and hassles. We studied the full document flow and the job of each and every employee and completely redesigned this process. We made it totally dedicated and optimized for pharmaceutical needs.
The result
The new solution was used for one of our early adopters in the UK and we helped this company to migrate all their documents and data into the new platform in the meantime polishing it and implementing feedback from employees. Stabilizing MVP and shifting all employees to the new principles took 6 months. This system awaits more investments now to proceed with development and sales.
Document storage and management
We have developed a database and storage for files and documents related to pharmaceutical products. During the development and life of a pharmaceutical product, the documentation is regularly updated and new ones appear, and all records are unique for each product. Consequently, this requires a lot of storage space.
From our side, we did everything from planning to implementation. The application provides an interface that allows you to access any of the files and watch all changes in the process of product development.
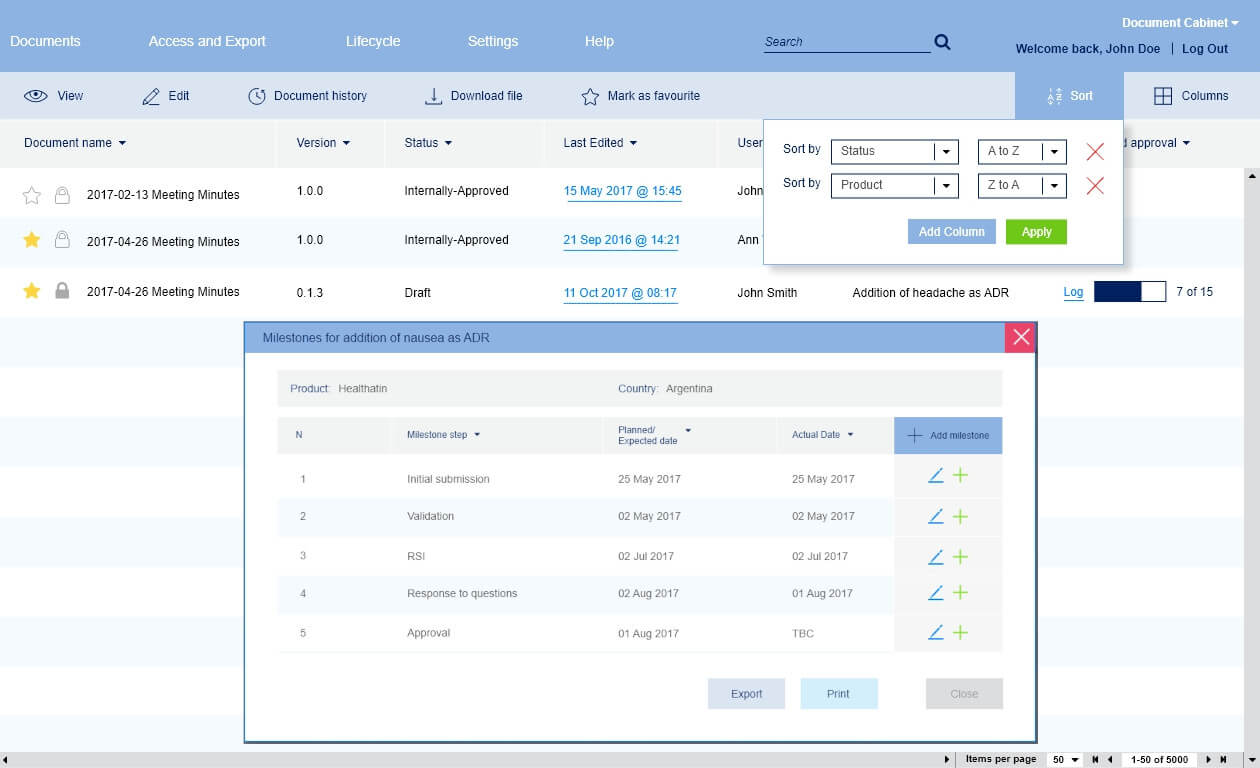
Version control and tracking
Each pharmaceutical product document has a large number of versions. This is due to the fact that everything in this document must undergo a certain verification by various medical structures. Because of this, a large number of versions arise.
We have developed a version structure specifically for pharmaceutical documentation since it is very different from other industries. Documentation change control is carried out using an interface where you can track the entire history.
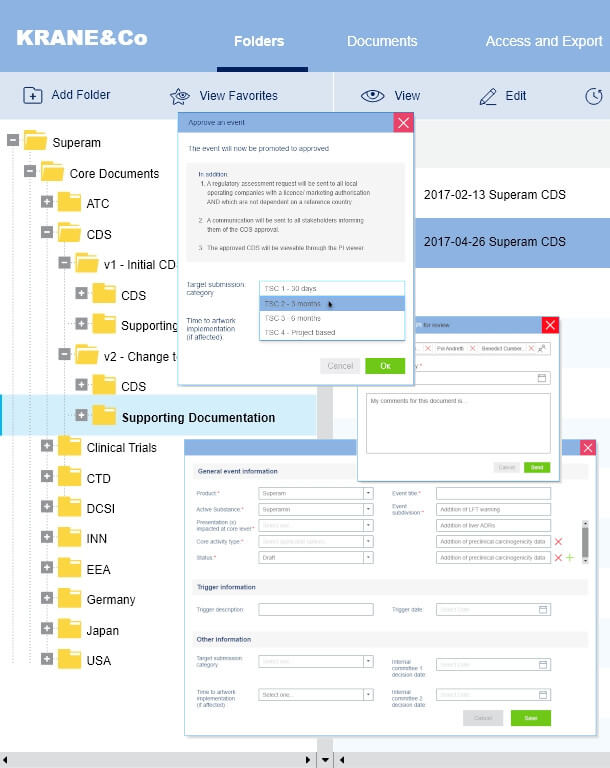
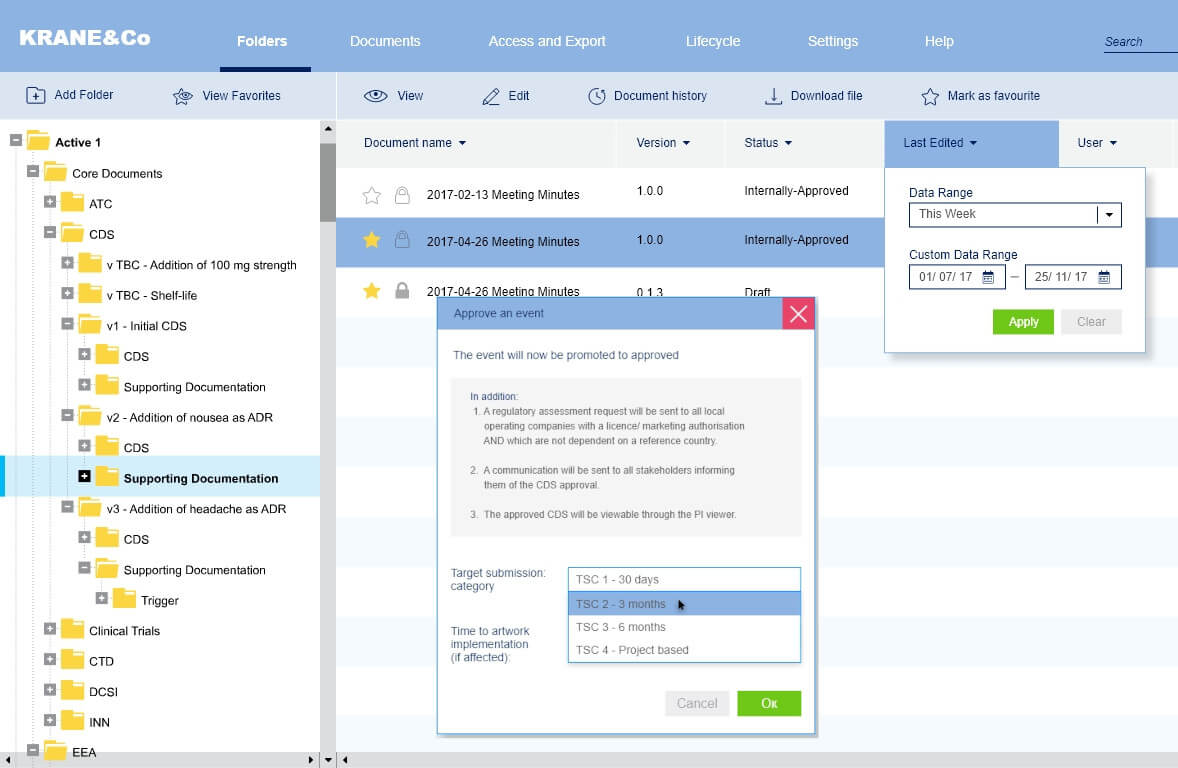
Approval process
We have solved the problem of updating the process of confirmation of pharmaceutical products since the standard CRM systems are not adapted to this unique process.
We have set up all the accounting for this process, including discussions, comments between the agents of this process, status updates, and the appointment of those responsible for the implementation of this process.
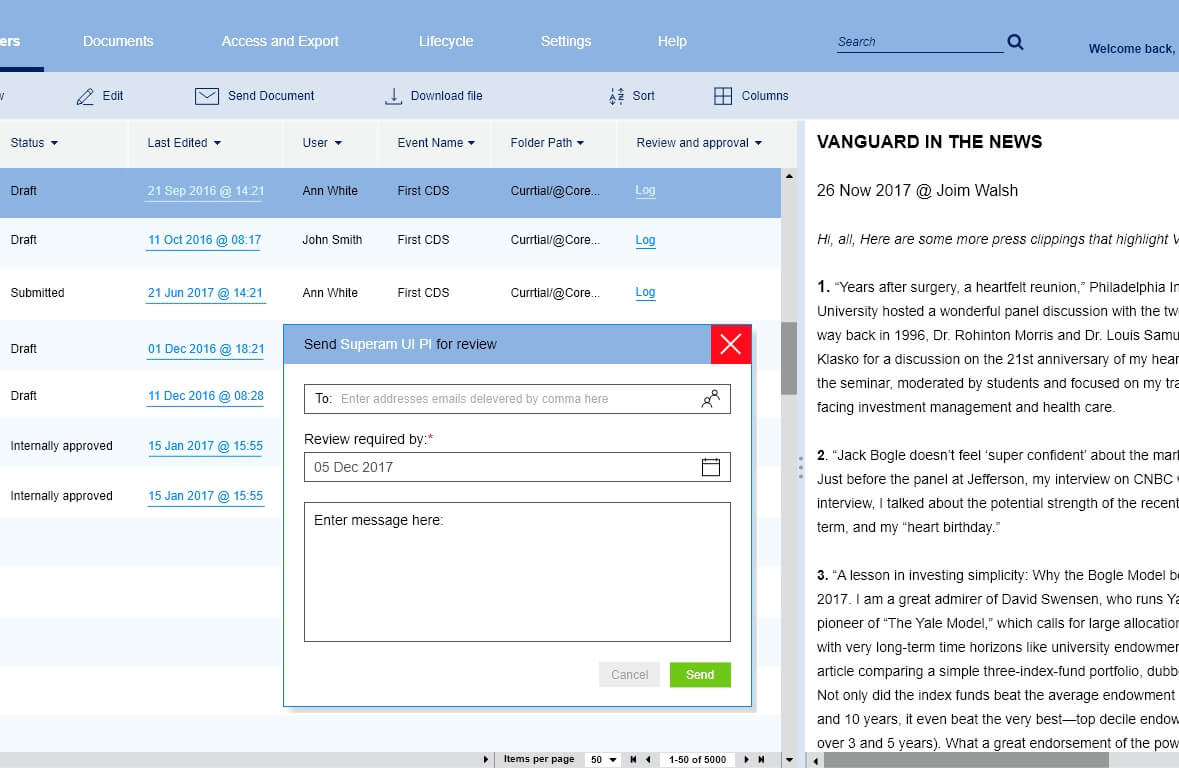
Version control and tracking
Each pharmaceutical product document has a large number of versions. This is due to the fact that everything in this document must undergo a certain verification by various medical structures. Because of this, a large number of versions arise.
We have developed a version structure specifically for pharmaceutical documentation since it is very different from other industries. Documentation change control is carried out using an interface where you can track the entire history.
Get Insights from Real SaaS Builds
Enjoyed the read? We write these case studies and articles to share what works — and what doesn’t — in real SaaS delivery. Got a challenge of your own? Let’s talk tech.

Ryzhokhin